Notes on Gabora 2013 -- Selectionism versus communal exchange
| Back to Essay List | Modified: 23 Jul 2020 | BibTeX Entry | RIS Citation |
This is a working draft, and sections may be incomplete. 6/15/13
Liane Gabora’s recent article “An evolutionary framework for cultural change: selectionism versus commual exchange” (Gabora 2013a), included invited comments by a number of researchers from a variety of fields, and a response by the author (Gabora 2013b). Carl Lipo and I wrote one of the comments in which we question Gabora’s basic claim that “culture does not evolve through a selectionist process” (Madsen and Lipo 2013).
Comments in Physics of Life Reviews are necessarily short – 3000 characters was the limit we were given. Our comment was thus quite telegraphic, listing the reasons why we believe Gabora’s claim is flawed, without supporting detail. These notes are the beginnings of an attempt to flesh out that argument. We feel this is important because Gabora’s response makes it clear that we are simply talking past one another, on an issue that is important to the study of evolutionary theory and the social sciences.
Is Gabora Redefining Natural Selection?
We stated that Gabora “adds unnecessary conditions to the requirements for selection.” Our rationale for this claim is simple: Gabora cites (with approval) John Holland’s proposition that systems evolving through natural selection share three “fundamental principles” (Holland 1975):
- Sequestration of inherited information
- Distinction between genotype and phenotype
- Mechanisms for discarding acquired information
The core of Gabora’s argument against natural selection’s applicability to cultural variation is that culture is a system of acquired phenotypic variation, and “natural selection only works as an explanation if acquired change is negligible.” The need for principles like the three proferred by Holland follows directly, since in her view selection can only proceed if acquired information can be held at bay.
We do not agree that the basic definition of natural selection as an evolutionary process does, or should, include conditions such as Holland lists, and Gabora adopts and advocates. The extent to which information acquired during an organism’s lifespan can be passed on to other organisms (including progeny) is a separate issue, one of the structure of transmission systems and their effects on the patterns seen in evolutionary histories. We return to this issue from an empirical perspective in the next section.
We certainly agree that “acquired” information can, in a theoretical model, dominate “inherited” information (depending upon their relative abundances and respective roles in development), and that one could see a loss of “phylogenetic signal” when attempting to determine taxonomic relationships based only upon the genetic component of an organism’s “genotype.” But if the issue is “what information goes into constructing an organism’s phenotype,” then we should be much less concerned about the distinction between “acquired” and “inherited” information since each are sources of causal influence on phenotypes. One of the most important contributions of “developmental systems theory” in the past decade has been a “demotion” of genetic information from the central role in constructing organisms and phenotypes, in favor of recognizing that every organism is built, survives, and exerts causal force on future generations through a variety of causal pathways, some genetic, some structural and epigenetic, some ecological, and even observational and linguistic (Jablonka and Lamb 2005; Oyama 2000; Oyama, Griffiths, and Gray 2003).
We see no reason to prejudge the question of whether selection can act effectively upon a particular mix of inherited and acquired information, given the recent literature on “inclusive inheritance” among scholars formulating the “extended evolutionary synthesis” (Bonduriansky 2012; Danchin et al. 2011; Day and Bonduriansky 2011; Mesoudi et al. 2013; Pigliucci and Müller 2010). The question, to us, asks not for an a priori answer which covers an entire inheritance system (e.g., human social learning, or human language), or an entire taxon (e.g., Homo sapiens), but specific answers for specific situations (we return to this point below). The efficacy of natural selection in the presence of mixed genetic, epigenetic, and cultural information is a theoretical and empirical matter. Such questions are solved by forming models of mixed inheritance systems, and then studying their fit to empirical data. Such models are under construction in the case of genetic and epigenetic mixes (Geoghegan and Spencer 2012), and have a long history in the study of cultural transmission (Boyd and Richerson 1985; L. Cavalli-Sforza and Feldman 1973; L.L. Cavalli-Sforza and Feldman 1973; Cavalli-Sforza and Feldman 1981; Lumsden and Wilson 1981; Pigliucci and Müller 2010).
Sequestration of Inherited Versus Acquired Information
Much of Gabora’s argument that selection cannot apply to human culture relies upon the lack of mechanisms in culture to partition “heritable” from “acquired” variation, given her belief that natural selection requires such a partition. In this section, we briefly review biological examples which suggest that natural selection requires no such partition. Evolution has constructed partitioning mechanisms, to be sure, but their presence in some taxa and not others is not an indication of where selection is present versus absent, but instead evidence of what kinds of circumstances favor sequestration, given its costs of construction and maintenance. We refer the reader to Leo Buss’s important book The Evolution of Individuality for more examples and a discussion of the evolutionary significance of sequestration mechanisms (Buss 1987).
Plants do not sequester germ line cells from the remainder of the somatic cells. Reproductive cells are constructed from somatic cells, usually at the vegetative meristem. The fact that plants can accumulate somatic mutations in branches, and then pass those mutations on to offspring organisms, has long been understood (Buss 1987). Oak trees, for example, are not genetically uniform but exhibit genetic mosaicism given different mutations in different branches. The older the oak tree, the greater the genetic diversity it possesses, and passes on seasonally through its acorns to daughter oak trees. In certain respects, each oak tree is a population of genomes, despite being a somatic individual with integrated physiology. Despite this flagrant display of seemingly “Lamarckian” incorporation of acquired variation into heritable variation, nobody has seriously suggested that oak trees, or plants in general for that matter, do not undergo natural selection. Plants comprise one of the major divisions of life on Earth, and some 350,000+ species, all of which evolve by natural selection, without sequestration of heritable information from environmental alteration.
Nor is this phenomenon limited to plants. The entire phylum Cnidaria, comprising over 10,000 species of jellyfish and corals, and the sponges (phylum Porifera), comprising over 9,000 species, do not possess specialized germ cells, and do not have any type of sequestration between somatic and heritable mutation (Buss 1987; Lieberman and Kaesler 2010). No one in the literature appears to be seriously suggesting that the lack of germ cells prevents sponges, comb jellies, and corals from undergoing natural selection.
The simple fact is that sequestration of specialized germ cells from the rest of the soma, to prevent accumulation of somatic changes to the DNA they carry, is a specialized adaptation that occurs in some major metazoan taxa (Buss 1987). Germ line sequestration is not a precondition for natural selection, but in fact is its product, constructed and maintained in some lineages but not others. When we claimed in our comment that “natural selection proceeds handily in many taxa which do not sequester heritable information,” it is to these 370,000+ species of life to which we were referring.
Nor is a lack of sequestration the only means by which acquired information can pass through reproduction to descendant organisms. Various forms of epigenetic inheritance, including DNA methylation sites and variation in chromatin packing levels, have been shown to be heritable and provide a route for acquired information to affect development in the next generation (see the excellent summary by (Jablonka and Lamb 2005)). Epigenetic inheritance systems (EIS) are common across animal and plant taxa, and operative in humans.
Simply put, it is not true that natural selection cannot occur in the presence of acquired variation. Among the Metazoa, one entire kingdom and two major animal phyla completely lack mechanisms for isolating a “germ line” from somatic variation. Nearly all Metazoa have one or more of the recognized epigenetic inheritance systems, suggesting that such mechanisms are ancient, generatively entrenched components of metazoan development. The inheritance by progeny of information acquired by organisms during ontogeny is a routine occurrence, despite the documented activity of natural selection in many lineages. Gabora is simply wrong that selection is incompatible with a mixture of acquired and inherited information.
Culture is nothing more than a set of genetically supported behavioral mechanisms by which information acquired during the lifetime of an organism can be shared with conspecifics. There is no a priori reason we can see that DNA methylation and chromatin marking would affect fitness and thus be subject to selection, but bird song acquisition or apprenticeship and teaching among humans would not.
This brings us full circle to our original claim in (Madsen and Lipo 2013): Gabora is attempting to show that natural selection is inapplicable to human culture, by adding unnecessary conditions for its operation to her definitions. Phenotypic variation among individuals is required for selection to occur; sequestration of a “germ line” of inherited genetic (or cultural) information is not.
Natural Selection and the Meaning of “Random Variation”
Nor does selection require that variation be generated “randomly.” It is certainly our understanding that the _teaching_of Darwinian evolution, from the Modern Synthesis through today, teaches that phenotypic variation is “random” or “blind” and that selection provides the directional force in evolution. Verbal descriptions of evolutionary theory and natural selection from Mayr to Dobzhansky to contemporary textbooks contain such descriptions (including the Hartl and Clark textbook that Gabora advised us to examine). And mathematical models of mutation often use random variables to generate new variants. But a balanced reading of the contemporary research literature reveals a much more interesting picture.
Let’s leave culture aside for the moment, and focus only upon biological systems and their variation, using the standard device of the “fitness landscape” (Gavrilets 2010; Wright 1932). And let’s start with mutations, which present the clearest case for random variation in evolution. Most mutations are produced randomly. Variants which arise through random nucleotide substitutions in a DNA sequence are random in two ways: the distribution of such mutations on the fitness landscape are described by a statistical distribution and are “isotropic” in “direction” on that landscape, and second, there is no causal coupling between the generation of such a mutant and the current gradient of fitness on the landscape.
Apart from simple mutations, the means by which genetic variation is generated is rarely “random” in the probabilistic sense. As is well known by now, developmental biology had little role in the Modern Synthesis, but since the 1980’s, an increasing focus on “evolutionary developmental biology” (or “evo-devo”) has revealed a host of details about how development generates phenotypic variation from genetic and epigenetic variants. Much of the phenotypic variation created by modifying the details of developmental events (e.g., timing, levels of expression) is highly structured in terms of “direction” in phenotype space but it is “uncoupled” from adaptive direction. In other words, the mechanisms of development can construct new variants which employ or “build upon” existing structures and thus are not “random” with respect to existing variants, but it is still the selective filter that determines which variants are adaptive. (Gerhart and Kirschner 2007; Kirschner and Gerhart 2005) refer to this as “facilitated variation,” which is probably a better term than “directed variation,” given the latter term’s history of referring to variation coupled to adaptation.
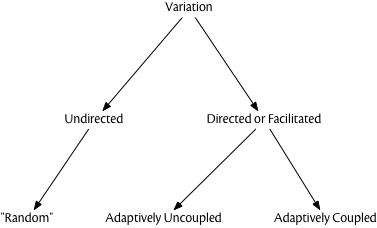
We’re not suggesting that most genetic variation is non-random, or that the standard pedagogical picture of “blind variation” and “directional selection” isn’t a useful way to teach the basics of Darwinian evolution. But simple descriptions of a complex theory hide detail and complexity, and it is increasingly clear that Darwinian natural selection can operate upon both random and non-random sources of variation. The “amount” of non-random variation in genetic inheritance may be smaller than with epigenetic or cultural inheritance, and probably is. Culture, in particular, is rife with mechanisms for “facilitated” variation, but planning, foresight, experimentation, and other such mechanisms are not necessarily coupled to adaptive outcomes (Mesoudi 2007). A “good try” is not the same thing as automatically detecting the optimal strategy or outcome.
Much of our evolutionary pedagogy does focus on randomness in the sense of direction, but it is the “coupling” between variation and fitness (or adaptive effect) that is crucial to distinguishing between Darwinian theory and the bundle of theories which go under the name “Lamarckian.” In developing the implications of Darwin’s theory of natural selection for generating adaptation out of the raw material of phenotypic variation, 19th and early 20th century Darwinians stressed the decoupling of the generation of variation and its selection (Toulmin 1972). The Lamarckian account of adaptation, in contrast, couples three elements to form a “one step” process. Variation is acquired during the lifetime of an organism and then is heritable in future generations, that variation is non-random but instead is “directed” towards better function, and finally the mechanisms of “direction” are coupled to the actual performance of function. Darwin himself recognized the possibility of the inheritance of acquired characters, but believed that the third element – adaptive coupling – was incorrect and was instead a two-step process, as do modern Darwinians (including ourselves). We have no trouble accepting that the “inheritance of acquired characters” occur in genetic evolution, and of course cultural transmission is nothing but a mechanism for the IAC, and we have no trouble accepting that some variation is generated non-randomly. We also have no trouble accepting that all such variation is then subject to natural selection.
Natural Selection: Force, or Statistical Consequence?
lorem ipsum sic dolor amet
Evolution as a Mix of Darwinian and Populational Processes…
lorem ipsum sic dolor amet
…And Cultural Evolution Even More So
lorem ipsum sic dolor amet
References Cited
Bonduriansky, R. 2012. “Rethinking Heredity, Again.” Trends in Ecology & Evolution. Elsevier.
Boyd, R., and P.J. Richerson. 1985. Culture and the Evolutionary Process. Chicago: University of Chicago Press.
Buss, Leo W. 1987. The Evolution of Individuality. Vol. 201. Princeton University Press Princeton.
Cavalli-Sforza, L., and M.W. Feldman. 1973. “Models for cultural inheritance. I. Group mean and within group variation.” Theoretical Population Biology 4 (1). Stanford Univ., CA: 42–55.
Cavalli-Sforza, L.L., and Marcus W. Feldman. 1981. Cultural Transmission and Evolution: A Quantitative Approach. Princeton: Princeton University Press.
Cavalli-Sforza, L.L., and M.W. Feldman. 1973. “Cultural Versus Biological Inheritance: Phenotypic Transmission from Parents to Children.(A Theory of the Effect of Parental Phenotypes on Children’s Phenotypes).” American Journal of Human Genetics 25 (6). Elsevier: 618–37.
Danchin, Étienne, Anne Charmantier, Frances A Champagne, Alex Mesoudi, Benoit Pujol, and Simon Blanchet. 2011. “Beyond DNA: Integrating Inclusive Inheritance into an Extended Theory of Evolution.” Nature Reviews Genetics 12 (7). Nature Publishing Group: 475–86.
Day, Troy, and Russell Bonduriansky. 2011. “A Unified Approach to the Evolutionary Consequences of Genetic and Nongenetic Inheritance.” The American Naturalist 178 (2). JSTOR: E18–E36.
Gabora, Liane. 2013a. “An Evolutionary Framework for Cultural Change: Selectionism Versus Communal Exchange.” Physics of Life Reviews 10 (2): 117–45. https://doi.org/10.1016/j.plrev.2013.03.006.
———. 2013b. “Reply to the Commentaries on an Evolutionary Framework for Cultural Change: Selectionism Versus Communal Exchange.” Physics of Life Reviews 10 (2): 162–67. https://doi.org/10.1016/j.plrev.2013.05.007.
Gavrilets, S. 2010. “High-Dimensional Fitness Landscapes and Speciation.” In Evolution: The Extended Synthesis, edited by Massimo Pigliucci and Gerd B. Muller, 45–79. MIT Press.
Geoghegan, J L, and H G Spencer. 2012. “Population-epigenetic models of selection.” Theoretical Population Biology.
Gerhart, John, and Marc Kirschner. 2007. “The Theory of Facilitated Variation.” Proceedings of the National Academy of Sciences 104 (Suppl 1). National Acad Sciences: 8582–9.
Holland, John H. 1975. “Adaption in Natural and Artificial Systems.” The University of Michigan Press.
Jablonka, E, and M.J. Lamb. 2005. Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral and Symbolic Variation in the History of Life. MIT Press.
Kirschner, Marc W, and John C Gerhart. 2005. The Plausibility of Life: Resolving Darwin’s Dilemma. Yale University Press.
Lieberman, Bruce S, and Roger L Kaesler. 2010. Prehistoric Life: Evolution and the Fossil Record. Wiley. com.
Lumsden, C. J., and E. O. Wilson. 1981. Genes, Mind and Culture. Cambridge: Harvard University Pres.
Madsen, Mark E., and Carl P. Lipo. 2013. “Saving Culture from Selection: Comment on an Evolutionary Framework for Cultural Change: Selectionism Versus Communal Exchange, by L. Gabora.” Physics of Life Reviews 10 (2): 149–50. https://doi.org/10.1016/j.plrev.2013.03.008.
Mesoudi, Alex, Simon Blanchet, Anne Charmantier, Étienne Danchin, Laurel Fogarty, Eva Jablonka, Kevin N Laland, et al. 2013. “Is Non-Genetic Inheritance Just a Proximate Mechanism? A Corroboration of the Extended Evolutionary Synthesis.” Biological Theory. Springer, 1–7.
Oyama, Susan. 2000. The Ontogeny of Information: Developmental Systems and Evolution. Duke University Press Books.
Oyama, Susan, Paul E Griffiths, and Russell D Gray. 2003. Cycles of Contingency: Developmental Systems and Evolution. Mit Press.
Pigliucci, Massimo, and Gerd B Müller. 2010. Evolution: The Extended Synthesis. MIT press.
Toulmin, Stephen Edelston. 1972. Human Understanding. Vol. 1. Princeton University Press Princeton, NJ.
Wright, Sewall. 1932. “The Roles of Mutation, Inbreeding, Crossbreeding and Selection in Evolution.” In Proceedings of the Sixth International Congress on Genetics, 1:356–66. 6.
| Back to Essay List | Modified: 23 Jul 2020 | BibTeX Entry | RIS Citation |
Tags
